- If Facility B enrolled in January 2021 and it’s currently December 2021, they can add monthly reporting plans and submit AU Option data back to January 2021.
- If Facility B enrolled in January 2020 and it’s currently January 2022, they can add monthly reporting plans and submit AU Option data back to January 2021.
Q6: How do I allow someone at my facility to view or upload data into the AU Option?
A user with administrative rights, such as the facility administrator, must follow the steps below to grant users access to the AU Option.
- From the NHSN Homepage, navigate to Users on the left-hand menu.
- Determine whether the user is new or existing within your NHSN facility.
- For people not already registered as users within your NHSN facility, select Add User. Complete all fields marked with a red asterisk (*) and select Save. Consider whether you should designate this new user as an AU Option and/or AR Option contact. The NHSN AUR Team uses this information to target notifications for AUR-related content like AU and AR quarterly users calls, updates regarding AUR resources, and AUR data quality outreach.
- For existing users, select Find User, locate the user, and click their name. Scroll down to the bottom of the page and click Edit, then click Edit Rights.
- On the next screen, assign the user rights and select Save. Users need rights to the Patient Safety Component to view and submit AU data. If the user will submit AU data, NHSN recommends Administrator-level user rights. You can use the Custom Rights option to limit user rights to just AU data and no other patient safety modules. Be careful when assigning Custom Rights, as certain settings will prevent users from accessing AU data. When assigning Custom Rights, users also need rights to the Patient Safety Annual Facility Survey to view Standardized Antimicrobial Administration Ratio (SAAR) reports, as the SAARs use variables from the Patient Safety Annual Facility Survey for risk-adjustment.
For complete details and instructions, please refer to the User Rights in NHSN – AUR Module document [PDF – 491 KB] .
Q7: Who can see my facility’s AU data in NHSN?
Once you upload data into NHSN, only users at your NHSN facility have access to view or edit your data unless one of the situations below applies. Users must generate data sets within NHSN data upload to view the AU data.
There is a special option for Groups (such as corporate healthcare systems or quality improvement organizations) to request rights to view your data, but you must accept the request before NHSN shares your data with them.
For the purposes of disease surveillance and prevention, state, local, and territorial health departments can have a special Data Use Agreement (DUA) with the CDC, which allows them to gain access to NHSN data from the facilities in their jurisdiction. Facilities in the state, local, or territorial health department’s jurisdiction are automatically added to a Group through the DUA process and health departments do not have to wait for facilities to accept the Confer Rights Template. You can find more information about the DUA process in the DUA Announcement.
In both cases, only users at the Group level can view your data. Facilities in the Group cannot view other facilities’ data. Additionally, Groups do not have the ability to add or edit your facility’s AUR data.
Further, NHSN does not share any AU Option data your facility submits to NHSN with CMS. The AUR measure included in the CMS PI Program is attestation-based so facilities simply attest yes or no to being in active engagement with NHSN.
Q8: How do I validate that my AU data are correct?
When you first start reporting data to the NHSN AU Option or change vendors, we recommend you use the Data Validation materials on the AUR Module webpage to perform an AU Option Implementation Data Validation [PDF – 459 KB] . These materials focus validation efforts on key AU Option protocol definitions and CDA requirements, including potential sources of error.
Additionally, we recommend you validate your AU Option data annually using the AU Option Annual Data Validation materials [PDF – 876 KB] . This validation is shorter and less comprehensive than the implementation data validation to reduce burden on facility staff responsible for AU Option reporting.
After importing AU data and generating data sets (see Data Import section of the AU FAQ), it is always a good idea to run the Antimicrobial Use Line List [PDF – 264 KB] and make sure what you submitted looks accurate.
Q9: How do I delete AU data?
You can delete AU data by following the steps below:
- From the NHSN Homepage, select Summary Data then Delete AUR Data on the left-hand menu.
- Select Antimicrobial Use Data as the Summary Data Type, then select the Location, Month, and Year you want to delete and click the Delete button.
Q10: How do I edit AU data I already imported into NHSN?
The NHSN AU Option does not allow manual data editing.
Many vendors offer a feature called succession management, which allows users to simply export a new version of files to NHSN. To update an existing record with new information, you can use succession management if your vendor offers this option. You can find additional details on succession management on the NHSN CSSP. Please note that succession management does not allow you to delete a record without overwriting it with a new record for that location/month.
If your vendor does not offer this feature, you can manually delete and re-upload the data by following the steps in question #9 in this section.
You must regenerate data sets within NHSN after uploading the corrected CDA file(s) to run analysis reports with the updated data.
Q11: When using Succession Management, can I delete records without replacing them?
No. Succession management will only overwrite or replace an existing record with a new record. If you need to completely delete an AU Summary record without replacing it with an updated version, you’ll need to follow the steps in question #9 in this section to manually delete the record.
Q12: I logged into my facility and can see that I have alerts for missing AU data. How do I get rid of them?
The missing summary data alerts mean that you included the AU Option in your monthly reporting plan but did not import these data into NHSN. You can clear the missing summary data alerts by:
- Submitting the AU data for those locations/months.
OR - Removing the AU Option for those locations from the monthly reporting plan(s).
The alerts are just a reminder and don’t affect your AU data in any way.
Q13: I’m from a health department. Could you tell me the names and NHSN orgIDs of the facilities submitting AU data in my jurisdiction?
Yes. NHSN’s updated Agreement to Participate and Consent allows NHSN to share specific information with state, local, and territorial health departments, Veterans Affairs (VA), and the Department of Defense (DoD) for prevention purposes, such as the NHSN orgIDs and names of facilities submitting AU and/or AR data within your jurisdiction. Please send your request to NHSN@cdc.gov.
For state, local, and territorial health departments that require this information on an ongoing basis, we suggest setting up a DUA with the CDC, which allows you to gain access to NHSN data from the facilities in your jurisdiction. Facilities in your jurisdiction are automatically added to a Group through the DUA process and you do not have to wait for the facilities to accept the Confer Rights Template. You can find more information about the DUA process in the DUA Announcement.
Q14: Does the Centers for Medicare and Medicaid Services (CMS) require reporting to the NHSN AUR Module?
Beginning in CY 2024, CMS finalized changes to the Medicare Promoting Interoperability Program for eligible hospitals and critical access hospitals (CAHs) that include a new AUR Surveillance measure under the Public Health and Clinical Data Exchange Objective. To obtain credit for calendar year 2024, eligible hospitals and CAHs must attest to being in active engagement with CDC’s NHSN to submit AUR data for the EHR reporting period, or else claim an applicable exclusion. Further, to meet the CMS PI Program requirement, facilities must use CEHRT updated to meet 2015 Edition Cures Update criteria, including criteria at 45 CFR 170.315 (f)(6).
Monthly Reporting Plan
Q1: How do I include the AU Option in my monthly reporting plan?
You need to add the AU Option to your monthly reporting plan for every month you plan to submit AU data. You cannot enter AU data “off-plan”.
- From the NHSN Homepage, select Reporting Plan from the left-hand menu.
- Click Add to add a new monthly reporting plan or click Edit to edit an existing plan.
- Select the month and year for AU data submission.
- If editing an existing plan, first scroll down to the bottom of the page and click Edit. Scroll to the Antimicrobial Use and Resistance Module section of the plan, enter all the locations for which you’ll submit AU data that month, and check the AU box (see screenshot below for reference). To include facility-wide inpatient (FacWideIN) in the monthly reporting plan for the AU Option, you MUST have at least one individual non-FacWideIN location (for example, medical ward, emergency department) added to the plan.
- Click the Save button at the bottom of the screen.
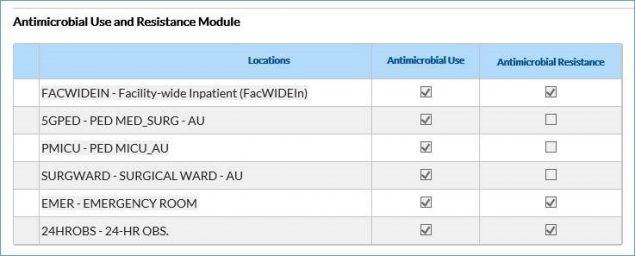
NOTE: You do not have to check the AR boxes unless you also plan to submit AR Option data.
Q2: Is a monthly reporting plan required for AU reporting?
Yes, the AU Option requires a monthly reporting plan with AU reporting indicated for every month you plan to submit AU data. You cannot enter AU data “off-plan”. See question #1 in this section for how to set up a monthly reporting plan.
- Non-FacWideIN locations can be the only location in the AU section of the monthly reporting plan.
- To include FacWideIN, you MUST have at least one individual non-FacWideIN location (for example, medical ward, emergency department) in the plan to save the monthly reporting plan.
Note: The AUR Measure of the CMS PI Program is attestation-based so CDC will not share AUR data with CMS, even if included in the monthly reporting plan. View the complete list of data required for each CMS Quality Reporting Program [PDF – 1 MB] .
Locations
Q1: What locations can I report AU data for?
NHSN strongly encourages the submission of data from all NHSN-defined inpatient locations (including procedural areas like operating rooms), FacWideIN, and select outpatient acute care settings (specifically, outpatient emergency department, pediatric emergency department, and 24-hour observation area) from which AU Option numerator and denominator data can be accurately captured. Your facility should not submit data from locations that cannot accurately capture electronic data. Additionally, your facility should exclude these locations’ data from the FacWideIN record.
Q2: Can I submit only FacWideIN data to the AU Option or do I have to submit location-level data as well?
You can submit only FacWideIN data to the NHSN AU Option; however, NHSN does not recommend doing this. When FacWideIN data are uploaded into NHSN, they represent aggregate data for all the inpatient locations reported by each facility and NHSN doesn’t have the ability to tease apart the data to separate it into specific locations. Your facility will be limited in the AUR Module features and Analysis Reports you can use by submitting only FacWideIN data. The SAARs are risk-adjusted using location-level information, so you will be unable to generate SAARs or use the Targeted Assessment for Antimicrobial Stewardship (TAS) reports and dashboards when submitting only FacWideIN data.
Additionally, you will not be able to save your monthly reporting plan with only FacWideIN and no other locations selected for the AU Option (see question #2 in the Monthly Reporting Plan section). Adding a location to the monthly reporting plan without submitting data from it each month will produce missing data alerts on the NHSN Alerts page. These alerts are just reminders and they do not affect your AU data in any way.
Q3: Should I include outpatient locations in the FacWideIN record for AU data submission?
No. For AU data submission, your facility should only include inpatient locations with electronically captured numerator (antimicrobial days) and denominator (days present and admissions) data in the FacWideIN record. Your facility should not include outpatient locations such as emergency department, pediatric emergency department, and 24-hour observation area in the FacWideIN record.
Q4: Should I count antimicrobials administered to observation patients in my AU data?
Your facility should include antimicrobials administered to all patients physically residing in an inpatient location at any time during a given month in the location-specific and FacWideIN CDA files, regardless of patient status (inpatient, outpatient, observation, etc.). While NHSN considers the 24-hour observation area location an outpatient location , not all facilities designate a separate physical location for observation patients. If your facility has a 24-hour observation area mapped in its NHSN Location Manager, you should not include antimicrobials administered to patients physically residing in the 24-hour observation area in the FacWideIN record. However, if your facility does not have a separate unit for observation patients, you may include their antimicrobial administrations in the location-specific and FacWideIN CDA files for the location in which they physically reside, regardless of patient status (inpatient, outpatient, observation, etc.).
Q5: Can I include AU data from the inpatient rehabilitation facility (IRF) and/or inpatient psychiatric facility (IPF) physically located in my hospital with the rest of my AU data?
You can include IRF and/or IPF AU data for IRFs/IPFs mapped as locations within your facility in NHSN. Your facility can also include the IRF/IPF location in your facility’s FacWideIN record. If your facility mapped the IRF/IPF as an NHSN location, you can add it to the AUR section of the monthly reporting plan regardless of whether it has a separate CMS Certification Number (CCN) from your hospital.
If your facility enrolled the IRF/IPF as a separate facility in NHSN (i.e., using a different orgID), you cannot report its AU data with the acute care hospital, but you can report it under the separate NHSN facility. The AU Option accepts data from an IRF or IPF enrolled in NHSN as its own facility using the rehabilitation hospital or psychiatric hospital designations, respectively. The AU data submission process for these hospital designations is no different than a general acute care hospital.
Q6: How should I account for locations that changed function during the COVID-19 pandemic in my AU data submissions?
NHSN provides general guidance for location mapping during the COVID-19 pandemic in the COVID-19 Location Mapping document [PDF – 173 KB] . However, because AU data are submitted electronically from vendor systems and it may not be possible to make certain changes within those systems, there are some additional considerations for reporting AU data. There are three options facilities can consider:
- Map temporary locations — Using the guidance linked above, you can work with your Infection Control/Infection Prevention department to determine if this is the right approach for your facility, as it will affect HAI reporting. Remapping in NHSN also requires mapping changes in your vendor system because the location information must be an exact match within both systems for AU files to successfully import into NHSN.
- Submit data to the locations as they are currently mapped — While this option doesn’t require remapping, facilities should use caution when interpreting SAAR values during the time the locations were affected by the COVID-19 pandemic because the SAARs are risk adjusted based on location designation. If the location designation doesn’t match the patient population of a given location, then any SAAR values involving that location will not be accurately risk adjusted. We recommend using AU rates instead of SAARs with this option.
- Submit data only for FacWideIN and outpatient locations — This option also doesn’t require remapping any locations but is not recommended. By not submitting location-specific data, you will not be able to generate SAARs, generate location-specific AU rates, or use the Targeted Assessment for Antimicrobial Stewardship (TAS) reports and dashboards.
Antimicrobial Days
Q1: Should I include topical, ophthalmic, and otic antimicrobial administrations in my AU data?
No. The AU Option only accepts four routes of administration: intravenous (IV), intramuscular (IM), digestive, and respiratory. Your facility should exclude any other routes of administration (for example, topical, antibiotic locks, intracavity, intrapleural, intraperitoneal, intraventricular, ophthalmic, otic, or irrigation) from AU Option reporting, including total antimicrobial days and sub-stratification of the routes of administration. Please refer to the spreadsheet titled SNOMED AU Routes of Administration within the AU CDA Toolkit for the complete list of eligible SNOMED codes and check with your vendor to make sure they only include the four routes listed above in your total antimicrobial days count.
Q2: If a patient receives two different antimicrobials in one day, does that count as one or two antimicrobial days?
The AU Option considers each antimicrobial separately. If a patient receives two different antimicrobials (for example, meropenem and amikacin) in the same calendar day, that patient contributes one meropenem antimicrobial day and one amikacin antimicrobial day to the location where the provider administered the antimicrobials.
See Appendix C of the AUR Module Protocol [PDF – 1 MB] for additional examples.
Q3: If a patient receives the same antimicrobial via two different routes in one day, does that count as one or two antimicrobial days?
If a patient is on a single antimicrobial (for example, ciprofloxacin) and the provider administers one dose in the morning via IV and another dose in the evening PO, that patient contributes one IV ciprofloxacin antimicrobial day and one digestive ciprofloxacin antimicrobial day. Because a single patient cannot contribute more than one antimicrobial day to the total antimicrobial days for each antimicrobial in a single calendar day, the patient only contributes one total ciprofloxacin antimicrobial day for each location.
See Appendix C of the AUR Module Protocol [PDF – 1 MB] for additional examples.
Q4: Providers administer vancomycin to patients in renal failure every other day but the drug stays in their system for two days. Does this count as one or two vancomycin antimicrobial days within the AU Option?
This counts as one vancomycin antimicrobial day because, even in the case of renal impairment, the AU Option only counts antimicrobials on the day of administration.
Q5: Why don’t the total antimicrobial days in my FacWideIN record equal the sum of antimicrobial days from all my location records?
The sum of location-specific antimicrobial days will always be greater than FacWideIN antimicrobial days because multiple administrations of the same antimicrobial in separate patient care locations within a single calendar day account for one antimicrobial day within each location but only one FacWideIN antimicrobial day. For example, if a patient received one dose of vancomycin in the medical ward and then transferred to the medical intensive care unit (ICU), where they received another dose of vancomycin on the same day, this patient contributes one vancomycin antimicrobial day to the medical ward and one vancomycin antimicrobial day to the medical ICU but only one vancomycin antimicrobial day to FacWideIN. Patients may only contribute one antimicrobial day to each location (including FacWideIN) per day.
Q6: The sum of the routes of administration in my AU line list does not equal total antimicrobial days for some antimicrobials. Why is this?
The sum of the four routes of administration for a given antimicrobial should always be greater than or equal to the antimicrobial’s total antimicrobial days. In cases where a provider administered the same antimicrobial more than once per day via multiple routes, the sum of the routes can be greater than the total antimicrobial days for that antimicrobial.
Keep in mind that the total antimicrobial days for a given antimicrobial should only include administrations via one of the four routes accepted into the AU Option (IV, IM, digestive, and respiratory). Your facility should exclude administrations via any other routes (see question #1 in this section for examples) from all AU Option data. Check with your vendor to make sure they only include the four routes listed above in your total antimicrobial days count.
Q7: Should incomplete antimicrobial administrations count towards antimicrobial days?
No. Your facility should not include incomplete antimicrobial administrations (for example, partial infusions) nor antimicrobials that were ordered but not administered. Your facility should only include completed administrations in AU Option data.
Days Present & Admissions
Q1: Is the days present denominator used in the AU Option the same as the patient days denominator used in other parts of NHSN?
No. The days present denominator is different from the patient days denominator. Days present are the number of patients present at any time on a given calendar day in each patient care location. Patient days are the number of patients present in each patient care location during the once daily census count.
For example as highlighted in the screenshot below, Patient A was admitted to the medical ICU at 00:01. They were transferred to the medical ward at 08:31. They stayed in the medical ward until they were discharged from the hospital at 24:00. Patient A would contribute one day present to the medical ICU and one day present to the medical ward because they were in each location at some point during that calendar day. Patient A would contribute one patient day to the medical ward because they were present in the medical ward at during the census at midnight. They do not contribute a patient day to the medical ICU because they were not present in the medical ICU at midnight during the once daily census.

Please note, the NHSN AU Option only counts a patient once for each location each day. For a given location or FacWideIN, the days present count should almost always be higher than the patient days count because days present take patient transfers and discharges into account while patient days do not.
Q2: Should my days present be equal to my patient days for a given location?
No. For a given location or FacWideIN, the days present count will almost always be higher than the patient days count because days present take patient transfers and discharges into account while patient days do not. On average, for all NHSN AU Option reporting hospitals, days present are 24% higher than patient days for the same month/location. There is some variation based on the location type. The percent difference is generally smaller in locations with longer lengths of stay and larger in locations with shorter lengths of stay. The table below provides the median percent difference between days present and patient days for select location types and the interpretation based on the average for all AU Option reporting facilities. NHSN calculated the percent difference with the following formula:
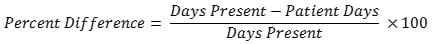
| Location Type | Median Percent Difference | Interpretation |
| FacWideIN | 24% | Days present are 24% higher than patient days for the same month/location at the FacWideIN level. |
| Adult ICUs | 25% | Days present are 25% higher than patient days for the same month/location in adult ICUs. |
| Adult Wards | 25% | Days present are 25% higher than patient days for the same month/location in adult ward locations. |
| Pediatric ICUs | 24% | Days present are 24% higher than patient days for the same month/location in pediatric ICUs. |
| Pediatric Wards | 35% | Days present are 35% higher than patient days for the same month/location in pediatric ward locations. |
| Neonatal ICUs | 8% | Days present are 8% higher than patient days for the same month/location in neonatal ICU locations. |
| Neonatal Step-Down Unit | 16% | Days present are 16% higher than patient days for the same month/location in neonatal step-down units. |
| Well Baby Nursery (Level I) | 34% | Days present are 34% higher than patient days for the same month/location in well baby nursery locations. |
| Labor, Delivery, Postpartum Units | 43% | Days present are 43% higher than patient days for the same month/location in labor and delivery locations. |
| Outpatient Locations (ED, Pediatric ED, 24 Hour Observation Locations) | 14% | Days present are 14% higher than encounters for the same month/location in outpatient locations. |
NHSN recommends validating your AU days present denominators using the methodology starting on page 10 in the AU Option Implementation Data Validation protocol [PDF – 1 MB] to ensure your vendor system aggregates them correctly, as they can influence your SAAR calculations.
Q3: If a patient transfers between locations in one calendar day, how many days present does that patient contribute to the FacWideIN record?
The patient contributes one day present to the FacWideIN record. The NHSN AU Option only counts a patient once per calendar day for the FacWideIN record.
Q4: Which patients are counted in the FacWideIN admissions?
Admissions are defined as the aggregate number of patients admitted to an inpatient location within the facility (FacWideIN) starting on first day of each calendar month through the last day of the calendar month. A patient is counted as an admission when they arrive in an NHSN designated inpatient location regardless of patient status (for example, inpatient, observation). A patient admitted to an inpatient location is counted as an admission even if they are discharged that same calendar day. However, a patient transfer from an inpatient to an outpatient location then back to an inpatient location is counted as two separate admissions.
Please note, the admissions definition used in the AUR Module is different than the definition used for the NHSN Multidrug-Resistant Organism (MDRO)/Clostridioides difficile Infection (CDI) Module.
Q5: How do I count patient admissions when a patient’s stay extends from one month to another?
The day a patient enters the door to a facility or location is the date of their admission to that facility/location regardless of patient status (for example, inpatient, observation). If the patient remains in an inpatient location and the facility does not discharge the patient, then that stay is all part of the same admission, no matter how long. A stay that continues across multiple calendar months, assuming the patient remains in an inpatient location the entire stay, is still only one admission counted in the month the patient was originally admitted.
If the patient is discharged and then returns on a separate calendar day, then count the patient as a new admission with a new admission date. If the patient is discharged and then returns on the same calendar day, do not count the patient as another admission.
Data Import
Q1: Can I enter AU data manually by hand?
No. Though some NHSN Modules allow for both CDA import and manual data entry by hand, the AU Option only accepts data via CDA import due to the amount of data submitted each month. Please review the Uploading CDA Files into NHSN Quick Learn video [Video – 12 min] for additional details.
Q2: My vendor asked me to provide my facility’s OID. What is an OID and where do I get one?
An Object Identifier (OID) is a unique identifier for your NHSN facility. The AU Option uses the OID in CDA files to identify which facility submitted the files. If your facility submits data to other NHSN Modules via CDA import, you may already have an OID.
To verify if your facility has or needs an OID, users with administrative rights can navigate through the NHSN application following the steps below:
- Navigate to Facility then Facility Info on the left-hand menu.
- On the Edit Facility Information page, you’ll find the OID section on the top right of the screen (see screenshot below).
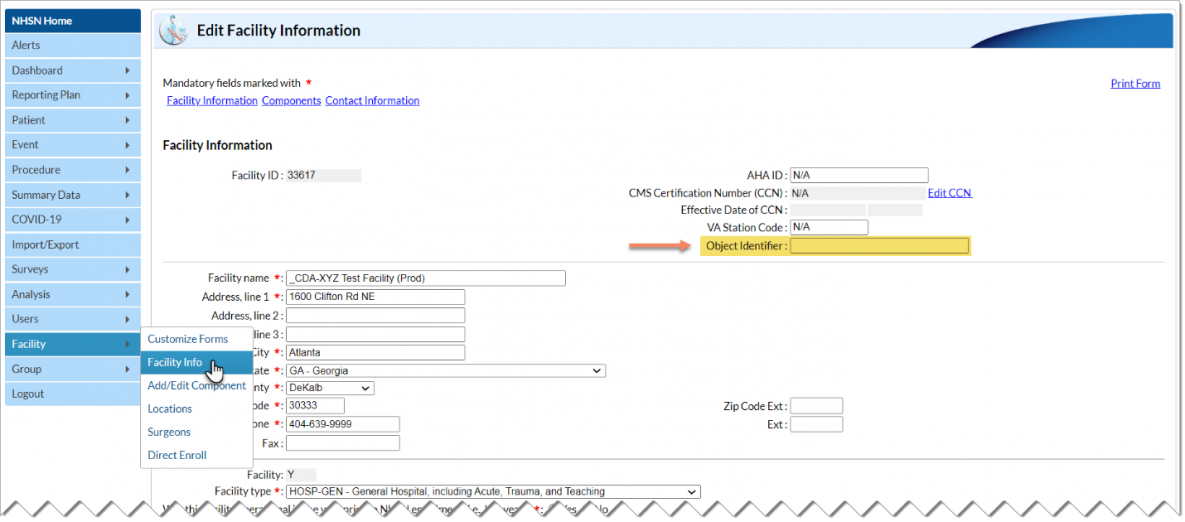
If you’ve verified that your facility does not have an OID, follow the steps outlined here to obtain one: Object Identifier (OID) Entry Procedure [PDF – 30 KB] .
Q3: Can I submit AU and AR CDA files together in the same zip file?
Yes, you can submit AU and AR CDA files together in the same zip file. For manual upload, all the CDA files in a zip file must be from one facility. For DIRECT submission, the zip file can contain CDA files for multiple facilities. For both manual and DIRECT submission, the zip file can contain up to 1,000 CDA files or a maximum of 2 MB, whichever comes first.
Please note: NHSN only accepts alphanumeric characters, hyphens, and underscores in CDA and zip file names. NHSN does not accept other special characters.
Q4: Do I need to include all antimicrobials that are required by the NHSN AU Option in my AU CDA file?
Yes, all required antimicrobials for the reporting calendar year must be included in the AU Option CDA files, regardless of whether your facility used all of them that month. Appendix B of the AUR Protocol [PDF – 1 MB] lists the required antimicrobials for the reporting calendar year. See question #5 in this section for how to report required antimicrobials that were not administered during a calendar month.
Q5: What do I do if I did not have at least one administration of all required antimicrobials each month?
You must report a value – a specific number, 0, or NA – for every antimicrobial listed in Appendix B of the AUR Protocol [PDF – 1 MB] , regardless of actual administration in your facility/location in a given month.
- Zero (0) — Use when your facility can electronically capture administrations of the antimicrobial in the eMAR/BCMA but did not administer it to any patients during the given month.
- Example: If your system can electronically capture amoxicillin via eMAR/BCMA but no providers administered it to patients during the month, the amoxicillin total antimicrobial days count should be 0.
- The CDA file expresses this as value=”0”.
- Example: If a provider gives amikacin via respiratory route to patients throughout the month, but the eMAR/BCMA cannot accurately capture the administrations, the amikacin antimicrobial day respiratory count should be ”NA”.
- Facilities should only use “NA” for non-formulary agents when those agents, if administered, cannot be accurately electronically captured. If use of non-formulary agents can be accurately electronically captured, no use of those agents in each location/month should be reported as “0” (zero).
- Facilities should use “NA” consistently across all locations and FacWideIN, and across months.
- The CDA file expresses this as nullFlavor=”NA” but the NHSN AU Option reports display NA as “.”.
Q6: I don’t see an option for uploading AU data. Please help!
In NHSN, after selecting Import/Export on the navigation bar, you should see “AUR Summary Data” or “Events, Summary Data, Procedures Denominators” as an option to upload CDA files [Video – 12 min]. Both options allow you to upload AU data into NHSN. If you do not see either of these options, a user with administrative rights must change your user rights. See the NHSN AUR User Rights [PDF – 350 KB] document for more information.
Q7: How do I know if all the CDA files I submitted together in the same zip file uploaded into NHSN successfully?
Sometimes, when uploading multiple AU files together, some files successfully upload and others do not. Here is a screenshot of what it looks like when you submit files together and some records pass but others fail. Note that both the Error Report and Submit buttons are enabled:
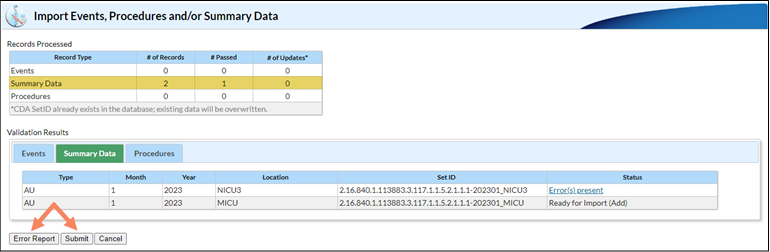
If you click on the Summary Data tab in the Validation Results table, you can see the files that passed and failed validation by looking in the Status column. In this instance, the user submitted two records – one passed validation and one failed. When they click the Submit button, only the file that successfully passed validation uploads to NHSN. Clicking the submit button generates a PDF report, which shows the file(s) that successfully imported and the file(s) that did not pass validation and did not import.
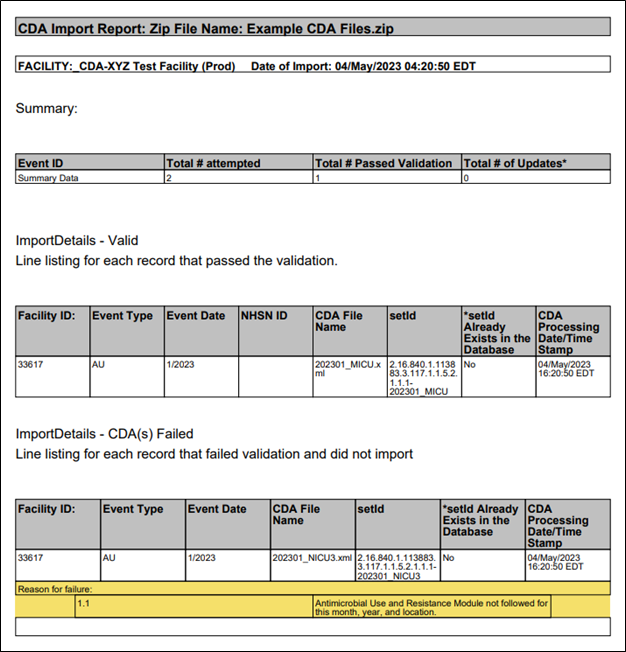
Please view and save the PDF report for your records each time you import data into the AU Option. The NHSN Help Desk requires information from the PDF report to assist you with inquiries related to data import errors.
Q8: I just uploaded data to the AU Option, but I don’t see it in the analysis reports. What happened to my data?
Newly uploaded data do not appear in the NHSN analysis outputs until you generate new data sets within NHSN. Your data set is a snapshot of the data currently in your NHSN facility during the time period specified when you click the “Generate Reporting Data Sets” button. Always generate new data sets after uploading data into NHSN. See the Generating Datasets Guide [PDF – 400 KB] for more information.
Note: Each NHSN user has their own data sets. You may not see the same data as your coworkers if you generated data sets at different times or with different parameters.
Q9: How do I view my AU data once I upload it into NHSN?
You can view AU Option data using the NHSN Analysis function. You can find details on AU Option analysis in the NHSN AUR Module Protocol [PDF – 1 MB] or in the Analysis Resources section of the NHSN AUR Module web page.
NHSN provides short analysis quick reference guides to assist with viewing, modifying, and interpreting AU Option data:
- Antimicrobial Use Line List [PDF – 300 KB]
- Antimicrobial Use Rate Table – By Location [PDF – 225 KB]
- Antimicrobial Use Rate Table – FacWideIN [PDF – 300 KB]
- Antimicrobial Use SAAR Baseline Rate Tables [PDF – 800 KB]
- Antimicrobial Use Bar Chart [PDF – 300 KB]
- Antimicrobial Use Bar Chart – Selected Drugs [PDF – 400 KB]
- Antimicrobial Use Pie Chart [PDF – 300 KB]
- Antimicrobial Use SAAR Table [PDF – 1 MB]
- Antimicrobial Use SAAR Table – By Location [PDF – 750 KB]
- Antimicrobial Use – SAAR Bar Chart by Location [PDF – 1 MB]
- Antimicrobial Use SAAR Plot [PDF – 1 MB]
- Antimicrobial Use – SAAR Bar Chart by Location [PDF – 1 MB]
You must generate data sets within NHSN after uploading new data to run analysis reports with the most current data. See question #8 in this section for more information about generating data sets.
Import Errors
Q1: When I try to upload my AU data, I get an error message that says “Please upload files with .zip extensions only. Try again.” What does that mean?
The NHSN AU Option requires users to upload CDA files in a zip file. Try zipping the file(s) and resubmitting. Note that each zip file can contain up to 1,000 CDA files or a maximum of 2 MB, whichever comes first.
Q2: When I try to upload my AU data, I get an error message that says, “Antimicrobial Use and Resistance Module not followed for this month, year, and location.” What does that mean?
This error message means that you did not add the month, year, and location in the CDA file you’re trying to upload in the Antimicrobial Use and Resistance portion of your Monthly Reporting Plan. You must add the location(s) to the Antimicrobial Use and Resistance portion of your Monthly Reporting Plan for every month you plan to submit AU data. NHSN will not accept out-of-plan data. To edit your monthly reporting plan to include AU, follow the steps in question #1 in the Monthly Reporting Plan section. After you edit your monthly reporting plan to include AU for the desired month, year, and location, NHSN should accept the CDA file.
If you verified this location is on your monthly reporting plan and NHSN still does not accept the file, incorrect location information in your CDA file may be causing the error. See question #3 in this section for details on location errors.
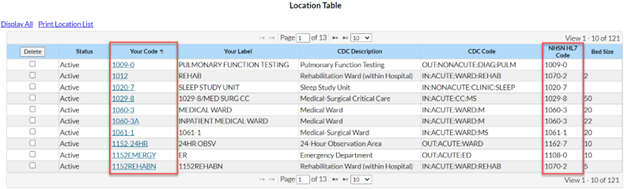
Q3: When I try to upload my AU data, I get an error message that says my location does not exist and I need to add it to my NHSN facility. I know my location is already mapped in NHSN. What does this error mean?
The location in the CDA file must be the exact match to the NHSN “Your Code” value, “NHSN HL7 code” and location type values in the NHSN Location Manager. This error message is telling you that the location name used in your CDA file does not match a location currently mapped in your NHSN facility.
We recommend meeting with your NHSN Facility Administrator to have them export the location list out of NHSN (Facility > Locations > Export Location List) so you can compare what’s in NHSN to what’s in your vendor system. Decide the best way to rectify the differences (update NHSN or update the vendor software) with your Infection Control/Infection Prevention department. Once you’ve matched the locations across both systems, re-export your AU CDA files and import them into NHSN.
Standardized Antimicrobial Administration Ratios (SAARs)
Q1: What is the Standardized Antimicrobial Administration Ratio (SAAR) and how is it calculated?
The SAAR is a metric developed by CDC to analyze and report summary antimicrobial use data. NHSN calculates the SAAR by dividing observed antimicrobial days by predicted antimicrobial days. More information on how NHSN calculates the SAAR can be found in the NHSN AUR Module Protocol [PDF – 1 MB] and the Guide to the SAAR [PDF – 1 MB] . Additionally, the Keys to Success with the SAAR webpage provides a short overview of the SAAR and its components, and SAAR training videos can be found under: NHSN AUR Training.
Q2: My facility has a SAAR over 1. Is that bad?
A high SAAR that achieves statistical significance indicates that location or group of locations used more antimicrobials than predicted. A SAAR that is not statistically different from 1.0 indicates antimicrobial use is equivalent to the referent population’s antimicrobial use. A low SAAR that achieves statistical significance indicates that location or group of locations used fewer antimicrobials than predicted. However, the SAAR alone is not a definitive measure of the appropriateness or judiciousness of antibacterial use. Any SAAR may warrant further investigation. For example, a SAAR above 1.0 that does not achieve statistical significance may be associated with meaningful antimicrobial overuse and require further investigation. Keep in mind, a SAAR statistically different from 1.0 may still not lead to productive investigation.
Q3: What locations are included in the SAAR?
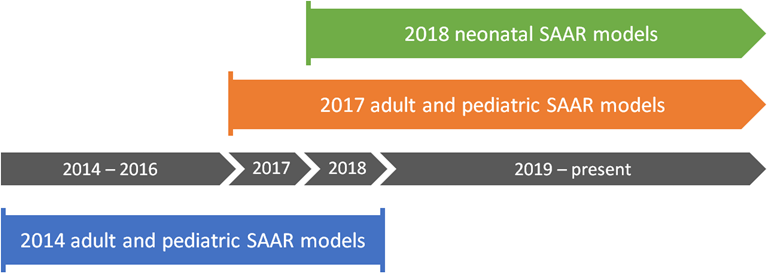
NHSN only generates 2017 baseline adult and pediatric SAARs for locations mapped using one of the following thirteen CDC location types:
- Adult Medical Critical Care
- Adult Medical-Surgical Critical Care
- Adult Surgical Critical Care
- Adult Medical Ward
- Adult Medical-Surgical Ward
- Adult Surgical Ward
- Adult Oncology General Hematology-Oncology Ward
- Adult Stepdown Unit
- Pediatric Medical Critical Care
- Pediatric Medical-Surgical Critical Care
- Pediatric Medical Ward
- Pediatric Medical-Surgical Ward
- Pediatric Surgical Ward
NHSN only generates 2018 baseline neonatal SAARs for locations mapped using one of the following four CDC location types:
- Special Care Nursery (Level II)
- Neonatal Critical Care (Level II/III)
- Neonatal Critical Care (Level III)
- Neonatal Critical Care (Level IV)
While NHSN encourages facilities to submit AU data from all NHSN-defined inpatient locations (including procedural areas like operating rooms), FacWideIN, and select outpatient acute-care settings (specifically, emergency department, pediatric emergency department, and 24-hour observation area), the only locations that can generate SAARs are those mapped to the CDC locations listed above. In the future, as more facilities submit AU data, NHSN hopes to develop SAARs for additional location types.
You can still examine antimicrobial use in other locations using line lists, rate tables, or charts. See question #9 in the Data Import section for more information on NHSN AU Option Analysis.
You can find more information about NHSN location mapping for the SAAR in Table 5 of the AUR Protocol [PDF – 1 MB] .
Q4: I uploaded FacWideIN AU data to NHSN but cannot generate a SAAR report. Why not?
FacWideIN is not a SAAR-eligible location. It is the aggregate of ALL individual inpatient locations. The FacWideIN record captures facility-wide data; specifically, data from all inpatient locations from which the AU numerator and denominator are captured. When FacWideIN data are uploaded into NHSN, they represent aggregate use for all inpatient locations reported by your facility. NHSN is not able to tease apart the FacWideIN counts to know how many antimicrobial days and days present came from each individual inpatient location.
The SAAR models were developed for specific patient care locations. FacWideIN was not included in the SAAR modeling because it includes different patient and location types mapped within NHSN for each facility. Please refer the list of locations in questions #3 in this section to see which adult, pediatric, and neonatal location types can generate SAAR reports. To generate a SAAR report, you must report data from at least one of the individual inpatient location types listed in questions #3 in this section.
Please refer to question #2 in the Locations section for additional information on limitations of reporting only FacWideIN AU data.
Q5: Why did my SAAR values change from the last time I looked at them?
NHSN uses data from the most recent Patient Safety Component Annual Hospital Survey for facility-level risk adjustment in the SAAR models. Once a facility completes the most recent survey and generates new data sets within NHSN, the SAAR reports update to use the most recent survey data for risk-adjustment. For example, prior to the completion of the 2022 survey, 2022 facility SAARs are risk adjusted based on the 2021 survey responses. After completion of the 2022 survey, the 2022 SAARs will be risk adjusted based on the 2022 survey responses.
It is possible that a facility’s survey responses moved them into a different risk adjustment category for one or more SAARs. The survey is due in March each year and it is normal to see small changes in the predicted antimicrobial days and SAAR values from what they were before the survey was completed. Please refer to the “Survey data and risk-adjustment” section of the SAAR Guide [PDF – 1 MB] for more information.
Q6: Can I generate a SAAR for just one location and one month?
Yes. You can generate a SAAR by month, quarter, half year, year, or cumulative time periods. You can generate a SAAR report for a specific location or location type. To generate a SAAR by location and month, follow the steps in the SAAR Table — by location Quick Reference Guide [PDF – 500 KB] . NHSN provides quick reference guides for each SAAR report on the Analysis Quick Reference Guides webpage.
Q7: Can I generate a SAAR for one specific antimicrobial?
No. Each SAAR includes a grouping of antimicrobial agents (with the exception of pediatric azithromycin and neonatal ampicillin, fluconazole, and vancomycin). NHSN designed the SAAR antimicrobial agent groupings to enable hospitals and other entities to assess progress toward antibiotic stewardship goals. These categories are generally mutually exclusive, meaning individual antimicrobials are found in only one SAAR category, except for the All antibacterial agents SAAR and the Antibacterial agents posing highest risk for CDI SAAR, which include antimicrobials found in other SAAR categories. The SAAR antimicrobial groupings are listed in Appendix E in the AUR Module Protocol [PDF – 3 MB] .
Q8: What years can I generate SAARs for?
NHSN currently offers three SAAR baselines, each applicable to a select set of patient care locations and time periods. You can generate historical 2014 baseline adult and pediatric SAARs for data between January 1, 2014 and December 31, 2018. You can generate 2017 baseline adult and pediatric SAARs for data from January 1, 2017 going forward. You can generate 2018 baseline neonatal SAARs for data from January 1, 2018 going forward. Please note: You cannot directly compare SAARs calculated under the 2014 baseline with 2017 baseline SAAR values.
To generate a SAAR by location and month, follow the steps in the SAAR Table—by location Quick Reference Guide. [PDF – 500 KB] .
Q9: I uploaded AU data to NHSN, generated data sets, and ensured my location is one of the SAAR locations, but I still cannot generate a SAAR report. What is going on?
You may not have the correct user rights to view the SAAR reports. NHSN uses the Patient Safety Annual Facility Survey for risk adjustment in the SAARs. Without access to the survey data, you cannot view the SAAR data. Review your user rights with your NHSN Facility Administrator and, once the Facility Administrator corrects your rights, generate new data sets. Refer to the guidance document [PDF – 350 KB] that outlines minimum AUR rights.
There are a few circumstances under which you cannot either generate SAAR reports or obtain a SAAR value for certain locations.
You will not be able to obtain a SAAR value in the following situations.
All SAARs:
- Locations reporting zero days present for the selected time period.
- Locations reporting more antimicrobial days than days present for any SAAR agent category (except the adult and pediatric All Antibacterial Agents SAARs).
- Locations with less than one predicted antimicrobial day.
You will not be able to generate SAAR reports in the following situations:
Adult SAARs:
Adult SAAR locations in a long-term acute care (LTAC) hospital, orthopedic hospital, psychiatric hospital, or rehabilitation hospital are not able to generate SAAR reports. The 2017 SAAR baseline adult referent population does not include these facility types.
Pediatric SAARs:
Pediatric SAAR locations in a critical access hospital, LTAC hospital, oncology hospital, orthopedic hospital, pediatric LTAC hospital, psychiatric hospital, rehabilitation hospital, surgical hospital, women’s hospital, or Veterans Affairs (VA) hospital are not able to generate SAAR reports. The 2017 SAAR baseline pediatric referent population does not include these facility types.
Neonatal SAARs:
- Neonatal SAAR locations in a critical access hospital, LTAC hospital, oncology hospital, orthopedic hospital, pediatric LTAC hospital, psychiatric hospital, rehabilitation hospital, surgical hospital, or VA hospital are not able to generate SAAR reports. The 2018 SAAR baseline neonatal referent population does not include these facility types.
- Facilities missing relevant Patient Safety Annual Survey data or facilities that indicate they do not care for neonates.
- Facilities reporting zero inborn and zero outborn admissions.
If you tried everything in the FAQ above and still can’t generate a SAAR, please email the NHSN Helpdesk at nhsn@cdc.gov.
Q10: How can I compare my SAAR values to other facilities?
The NHSN AU Data Reports provide summaries of SAAR distributions and percentages of use within SAAR antimicrobial agent categories in adult, pediatric, and neonatal locations. The SAAR distributions can help inform stewardship efforts by enabling hospitals to see how their SAARs compare to the national distribution. Facilities can use distributions to help set facility-specific SAAR goals. The percentage of AU by class and drug within a SAAR antimicrobial agent category provides insight into prescribing practices across differing patient locations, such as medical critical care units compared to medical wards. Facilities may evaluate these usage patterns in context of their local treatment guidelines, antimicrobial resistance rates, and formulary.
Q11: How do I figure out whether my SAAR values are going up or down over time?
Tracking AU over time using SAARs should be limited to comparing two SAAR values across two points in time. You can find more information about comparing two SAAR values in NHSN’s Guide to the SAAR [PDF – 1 MB] . You can also visually assess SAAR values over time to get a sense of whether values increased or decreased during that time period using the SAAR Plot report [PDF – 661 KB] .
However, you should not use SAARs for trend analyses, where SAAR values are simultaneously compared across many points in time to determine whether there is a statistically significant change in AU.
Q12: Why can’t I use SAAR values for trend analysis?
Trend analyses, where SAAR values are simultaneously compared across many points in time to determine whether there is a statistically significant change in AU, may violate the proportionality assumption that the SAAR models rely on. When statistically (as opposed to visually) comparing any pair of SAARs, the comparison is most valid when the constant proportionality assumption is satisfied. Specifically, when the distribution of exposure among the model strata is proportional to that of the referent population. Please see NHSN’s Guide to the SAAR [PDF – 1 MB] for more information about scalability of the SAAR and the proportionality assumption.
For example, let’s say in the referent population for the pediatric antifungal SAAR model, the proportion of days present (exposure) was 80% in pediatric ICUs and 20% in pediatric wards. The SAAR models assume other facilities not in the referent population have a similar distribution of days present. If one facility has 50% of its days present in pediatric ICUs and 50% in pediatric wards, comparisons can still be made, but there is a higher chance that changes in SAAR values over time are due to this difference in exposure rather than due to other factors such as stewardship interventions. As the distribution of the exposure shifts over time, then chances of violating the constant proportionality assumption increase.
If you are comparing SAARs within one facility and the days present distribution does not change much across the factors in the model and your time period of interest, it may be fine to make pair-wise comparisons of SAARs across various time points (2017 vs. 2018, 2018 vs. 2019, 2019 vs. 2020 etc).
You can also use rates for trend analyses to determine whether antimicrobial use has increased or decreased in a statistically significant way over your time period of interest. If you are assessing rates across various hospitals (rather than just within your own facility), you can work with a statistician to include risk-factors from SAAR models as factors in the trend analysis.
Q13: I’m not submitting data into the AU Option, but I’d like to compare my antimicrobial use against other facilities using the SAAR. Is this possible?
NHSN only makes the SAAR available for facilities reporting into the AU Option. NHSN published papers on the 2017 SAAR methodology and baseline model details (NHSN SAARs: A Progress Report and Risk Modeling Update Using 2017 Data ) and 2018 neonatal SAAR methodology (National Healthcare Safety Network 2018 Baseline Neonatal Standardized Antimicrobial Administration Ratios ) for reference.
Keep in mind, facilities not participating in the NHSN AU Option must still capture the NHSN AU Option-defined antimicrobial days and days present accurately to calculate their own SAARs using NHSN’s SAAR models.
Q14: Does the SAAR account for changes in antimicrobial use during the COVID-19 pandemic?
No. The AU Option does not collect patient-level information, including information on indication for use, co-morbidities, or severity of illness, nor does NHSN collect data on hospitals’ burden of COVID-19. Because of this, the SAARs are not adjusted for COVID-19 rates.
It is also possible that NHSN patient care locations changed function during the COVID-19 pandemic. These location changes may not be reflected in the SAARs because hospitals may not have remapped their patient care locations within NHSN due to the ongoing public health emergency. See question #6 in the Locations section for additional information on location changes during the COVID-19 pandemic.
The 2020 and 2021 NHSN AU Data Reports provide additional insight on AU rates during the COVID-19 pandemic.
Targeted Assessment for Stewardship (TAS)
Q1: What is the Antimicrobial Use Cumulative Attributable Difference (AU-CAD) and how do I interpret it?
The AU-CAD is the number of antimicrobial days needed to achieve a desired SAAR target. The higher the AU-CAD value, the greater the number of antimicrobial days that need to be reduced to meet the SAAR target.
NHSN calculates the AU-CAD by multiplying a numerical SAAR target value by predicted antimicrobial days and then subtracting that from observed antimicrobial days, rounded to the nearest whole number.
AU-CAD = Observed antimicrobial days - ( Predicted antimicrobial days × SAAR target )
A positive AU-CAD value can be interpreted as the number of antimicrobial days to reduce per time period to decrease the SAAR value and reach your SAAR target. A negative AU-CAD value can be interpreted as the number of antimicrobial days to add per time period to increase the SAAR value and reach your SAAR target.
Q2: How do I choose a SAAR target?
There are many factors to consider when defining target SAAR values. Unlike the Healthcare Association Infection (HAI) Targeted Assessment for Prevention (TAP) Strategy and Standardized Infection Ratios (SIRs), there are no national SAAR goals. Higher SAARs might reflect opportunities to improve use, but these values might be justified in some instances. Additionally, a SAAR value of 1.000 does not necessarily mean AU is ideal. Clinical judgement and patient information should be considered when interpreting SAAR values and setting SAAR targets.
SAAR target values may differ among facilities based on factors such as the original SAAR value for a given SAAR agent category, stewardship priorities, and clinical relevance. Additionally, SAAR target values may differ among SAAR agent categories and/or populations (adult, pediatric, neonatal) within a given facility.
The TAS Guide [PDF – 2 MB] contains resources to help you select SAAR targets.
Q3: Can my SAAR target be zero?
No, zero is not an appropriate SAAR target. While working towards zero healthcare associated infections is a meaningful goal, zero antimicrobial use is not the goal of antimicrobial stewardship. Stewardship interventions focus on reducing inappropriate antimicrobial use not eliminating antimicrobial use altogether.
Q4: How do I set a SAAR target within NHSN?
You have to enter SAAR targets within the NHSN application before using the TAS reports or TAS dashboard. These SAAR target values will be saved and used each time you run TAS reports and TAS dashboard until you manually update the values. You can update your SAAR target values at any time by following the same steps.
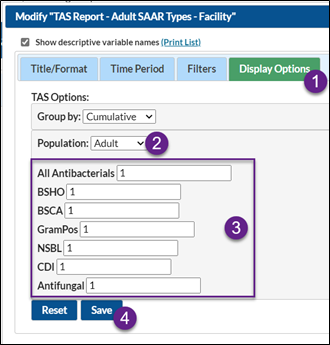
To enter your SAAR target values within the TAS Reports, select any TAS report and click Modify Report.
- On the Modification Screen, click the Display Options tab (see below screenshot)
- Note the population (adult, pediatric, or neonatal)
- Enter each SAAR target value
- After entering your SAAR target values, click Save
To enter your SAAR target values from the TAS dashboard, navigate to the TAS dashboard by selecting “Dashboard” from the lefthand menu in NHSN, and then “TAS Dashboard”.
- Note the population (adult, pediatric, or neonatal)
- Enter each SAAR target value
- After entering your SAAR target values, click Save
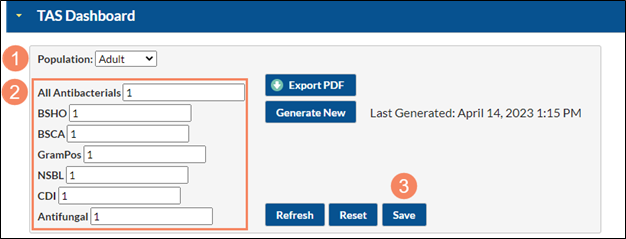
Please be sure to enter a SAAR target for each population and SAAR Type. Click Save after entering the SAAR target values for each population.
Q5: How do I determine which TAS report to use?
The Keys to Success with TAS webpage provides a flow chart and questions to help you choose which TAS report fits your needs. You can read more about each of the reports in the TAS Guide [PDF – 2 MB] and TAS quick reference guides.
Q6: What is the difference between the TAS reports and TAS dashboard?
The TAS reports and TAS dashboard are both tools to help stewards identify where stewardship efforts may have the greatest impact.
The TAS reports display AU-CADs for the most recent complete 12 calendar months at the group, facility, location group, and location level. The TAS reports are modifiable and allow you to drill down AU-CAD values by a variety of factors.
The TAS dashboard allows you to visualize AU-CAD values over time by quarter for the most recent complete four calendar quarters at the group, facility, and location level. Unlike the TAS Reports, the time period and level of aggregation displayed by the TAS Dashboard cannot be changed.
Q7: How many months of data are included in the TAS reports? Can I include more months?
By default, the TAS reports use data reported from the most recent 12 calendar months. Specifically, a TAS report run on July 15, 2022, will automatically include all reported AU data from July 2021 through June 2022. You can modify the output to display tables according to calendar year, half-year, quarter, or month within that 12-month span. For example, a TAS report run on July 15, 2022, using the modification to “group by summaryYQ” would produce 4 tables for the quarters in the 12-month span between July 2021-June 2022 (Quarter 3, 2021, Quarter 4, 2021, Quarter 1, 2022, and Quarter 2, 2022). You cannot modify the TAS reports to include additional months outside of the most recent 12 calendar months.
Q8: What locations are included in the TAS reports and TAS dashboard?
The TAS reports and TAS dashboard include only those location types that can generate SAARs; in other words, the same locations in your SAAR reports will also be included in the TAS reports. See question #3 in the SAARs section for more information on which locations are included in the SAARs.